Contributor: Julie Phillips, CHIM, CTR
NSPE Chapter

If you’ve ever wondered what a Certified Tumor Registrar (CTR) does, read on for a snapshot of this role within the Prince Edward Island Cancer Treatment Centre (PEICTC).
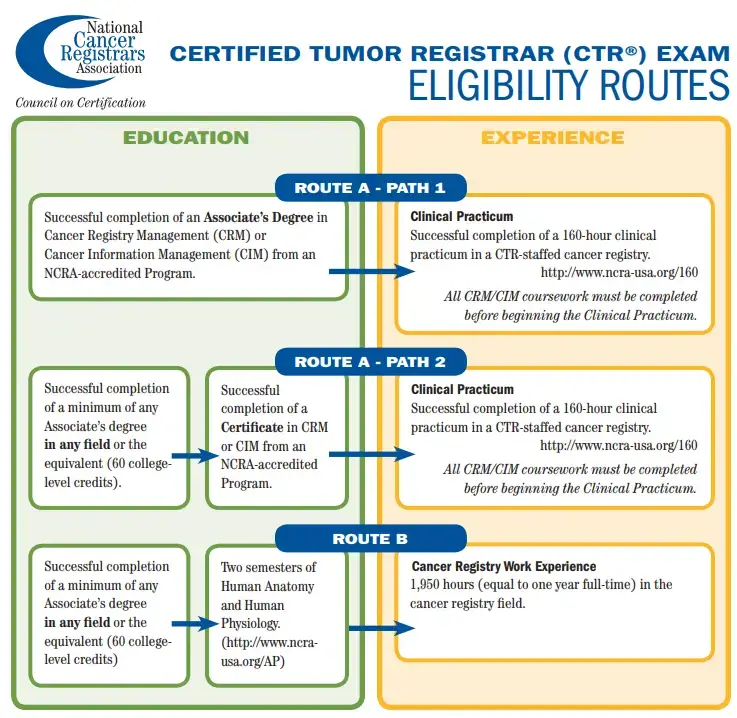
Like all health information professionals, those who want to specialize as Certified Tumor Registrars (CTR) begin with education in health information management (HIM). Many become certified in HIM with the Canadian College of Health Information Management, holding a CHIM designation after passing the CHIM national certification examination (NCE).
An aspiring CTR would specialize from there: training for one or two years in health information that is exclusive to oncology. Becoming a CTR requires writing and passing a national certification examination offered by the National Cancer Registrars Association (NCRA), which is based in the United States. CTRs have their own set of continuing education requirements, based on a two-year cycle.
Cancer registries collect information on cancer incidence, stage at diagnosis, and cancer treatments, and the PEI cancer registry is no different in this regard. The registry reports on cancer diagnoses for all Prince Edward Island (PEI) residents and records all cancer treatments, including surgeries, radiation therapy, and systemic therapy.
While the PEICTC operates as a hybrid system—using both electronic and paper charts—almost all required information can be located electronically. When abstracting, staging, and adding treatment information for a reportable neoplasm, the CTR accesses a person’s electronic health records (EHR) on the Cerner EHR platform to obtain demographic information, pathology reports, imaging reports, consult reports, and any other relevant information. To capture systemic therapy and radiation therapy information, the registrar uses the ARIA Oncology Information System. In order to obtain a complete picture of a person’s health history, paper charts are consulted in conjunction with EHRs—the former being especially helpful when capturing diagnostic, staging, or treatment procedures that were completed outside of the province. If further information is required beyond the electronic and paper records that are available, the CTR may also contact different facilities or physicians to gather more. All information is then captured in a system called OncoLog.
When it comes to coding neoplasms, the CTR follows the lead of the Canadian Cancer Registry (CCR), the organization that determines which neoplasms are reported in Canada. Another key coding tool in the CTR’s tool belt is the International Classification of Diseases for Oncology (ICD-O), which guides coding for the primary site, histology, and behaviour of reportable neoplasms. Here’s a brief overview of what codes look like with regards to oncology: the first four digits of the morphology code represent the histology of the neoplasm, and the fifth digit represents the behaviour. Behaviours of interest include those representing benign neoplasms (/0); those for borderline (/1); those for in situ neoplasms (/2); and those for malignant neoplasms (/3).
Incidence data is another important area of reporting that is in the CTR’s purview, including date and method of diagnosis (e.g., cytology, histology, imaging, or death certificate only); diagnostic confirmation method; primary site; morphology, grade, laterality; and whether there is lymph vascular invasion. Some of the staging data typically reported includes these categories: tumor (T); regional lymph nodes (N); distant metastasis (M); and different site-specific data items, which vary depending on the neoplasm being staged. Presently in Canada, the CTR is responsible for reporting the appropriate clinical stage and pathological or post-neoadjuvant therapy pathological stage, with each primary neoplasm being assigned two stage groups: clinical (c), and either a pathological stage (p) or a post-therapy stage (yp).
In order for registrars to identify appropriate primary sites, histology, and the number of primary neoplasms a person has, CTRs consult the Surveillance, Epidemiology, and End Results (SEER) program. For guidance on coding for the number of primary neoplasms, primary sites, and morphology, SEER references are consulted. They include: the 2018 Solid Tumor Rules manual, for any solid tumors diagnosed as of January 1, 2018; and the Hematopoietic and Lymphoid Neoplasm Database (HEME DB), for hematopoietic and lymphoid neoplasms. For guidance on staging, Canadian CTRs currently consult the 8th edition of the American Joint Committee on Cancer (AJCC). When there are discrepancies between SEER and CCR reporting standards, CTRs in Canada follow the latter.
CTRs play a key role in documenting all aspects of occurrence and care in oncology—information that is essential to everything from cancer research to the development of treatment programs. For health information professionals looking to specialize in the direction of oncology and Canada’s cancer registries, interesting careers await.
Relevant websites:
- Becoming a Certified Tumor Registrar (CTR): https://www.ncra-usa.org/Portals/68/PDFs/CTR_Eligibility_Graphic.pdf?ver=2017-07-23-164454-277
- American Joint Committee on Cancer (AJCC): https://www.facs.org/quality-programs/cancer/ajcc
- Canadian Cancer Agency (CCR): https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&Id=1215604
- Hematopoietic and Lymphoid Neoplasm Database (HEME DB): https://seer.cancer.gov/seertools/hemelymph/
- International Classification of Diseases for Oncology: http://www.iacr.com.fr/index.php?option=com_content&view=category&layout=blog&id=100&Itemid=577
- National Cancer Registrars Association (NCRA): https://www.ncra-usa.org/
- Surveillance, Epidemiology, and End Results (SEER): https://seer.cancer.gov/



